Phosphorus recovery by sludge acidification and dewatering

Cejna Anna Quist-Jensen1, Lisbeth Wybrandt1, Hanne Løkkegaard2, Sebastian Buch Antonsen2, and Morten Lykkegaard Christensen1*
1Aalborg University, Fredrik Bajers Vej 7H, 9220 Aalborg Ø, Denmark
2Danish Technological Institute, Kongsvang Allé 29, 8000 Aarhus C, Denmark
*Corresponding Author: mlc@bio.aau.dk
A pilot-scale Danish study of phosphorus extraction from acidified anaerobic and aerobic sludge samples has revealed removal to be more effective from anaerobic sludges, even though the digested sludge alkalinity increases the total acid demand
1. Introduction
Currently, the recovery and reuse of phosphorus (P) from wastewater is only considered viable for the return liquors from digestion or dewatering operations. However, it may also be extracted directly from sludge by acidification.
P exists in different forms in sludge, namely:
- chemically-bound
- organically-bound
- as polyphosphate in polyphosphate-accumulating organisms (PAOs) for biologically-removed P.
More than 80% of the P can be dissolved by acidification, depending on the sludge type, e.g. digested, undigested, primary, WAS, etc. The P concentration is lower in undigested sludge, and less of it is released, but the required sulfuric acid concentration for P extraction is higher for digested sludge due to the higher alkalinity levels.
For the following study, both digested and non-digested sludge were tested for P recovery by acid extraction, based on a pilot-scale study.
2. Materials and methods
Sludge was sampled from three Danish wastewater treatment plants (Table 1):
- Randers WwTP, which employs ferric chloride dosing and biological phosphorus removal based on a two-step ASP
- Kolding WwTP, which employs both biological (PAOs) and chemical processes
- Haderslev WwTP, which employs a single-step activated sludge process (ASP) with ferric chloride dosing and biological phosphorus removal.
| Parameter | Randers | Kolding | Haderslev2 |
|---|---|---|---|
1Digested (mesophilic) and centrifugally dewatered sludge | |||
| Capacity, p.e. | 160,000 | 125,000 | 56,000 |
| % operating capacity | 50% | 72% | − |
| P in, mg/L | 6.78 | 6.20 | 7.75 |
| P out, mg/L | 0.42 | 1.03 | 0.45 |
| Sludge composition | |||
| Dry matter, DM, g/kg sludge1 | 27.1±0.7 | ||
| Alkalinity, mgCaCO3/L (meq/L) | 6400−6800 (128−136) | ||
| S content, gS/kg DM | 11.2 | ||
| AD conditions | Not used | ||
| Sludge origin | 1° & 2° | 1° | − |
| HRT, d | 33−37 | 30 | − |
| Polymer | Bofloc D 6264 K | Neroplan CE1587 | Praestol K232L |
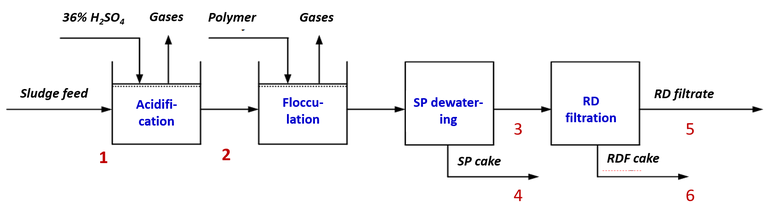
A 1 m3 pilot plant was set up at the sites for sludge acidification (with 36% H2SO4) and separation using a screw press (SP) (Fig. 1). At the Randers and Kolding sites, tests were conducted at adjusted pH values of 2, 3 and 4, as well as reference tests without acidification, whereas at Haderslev a single pH value of 3 was used. At Randers four extra experiments were conducted at pH 3 to explore the process in more detail. The reference tests employed the polymer reagent used at each site prior to the dewatering stage, whereas polymers more appropriate to the prevailing low-pH conditions were used for the acidified sludge samples.
Scoping trials revealed a 30-minute period as being the optimal reaction time for the acidification reactor to attain an equilibrium pH. Around 400 mL of defoamer was added to the reactor to suppress foam formation due to the impact of acidification.
Following acidification the sludge was flocculated with polymer and dewatered, with the mass flow of SP filtrate and dewatered sludge cake continuously monitored. The SP filtrate was filtered to 200 µm with a drum screen to remove excess organic matter. Total phosphorus (TP), orthophosphate (OP) and dry matter (DM), along with conductivity and metals content (Fe, Ca and Mg), were measured at six sample points in the process treatment scheme (Fig 1).
3. Results
Analysis of the sludge from the three sites (Table 2) indicated the conductivity and OP and dissolved P concentrations to be much lower for the non-digested sludge of Haderslev than for the digested sludge samples from the two other sites. This was to be expected, given the impact of digestion on organophosphate degradation and subsequent inorganic phosphate release.
| Parameter | Randers | Kolding | Haderslev |
|---|---|---|---|
*acidified sludge | |||
| Dry matter | 35.9±0.6 g/kg | 21.30±0.05 g/kg | 22.7 g/kg |
| Organic content | 56.3±0.6 % | 64.0±0.7 % | 65.6 % |
| Total phosphorus, TP | 38.1±0.4 g/kg DM | 21.6±0.9 g/kg DM | 28.7 g/kg DM |
| Orthophosphate, OP | 2.3±0.5 g/kg DM | 2.1±0.6 g/kg DM | 0.2 g/kg DM |
| Dissolved phosphate | 6% | 10% | 0.6% |
| pH | 7.2±0.1 | 7.10±0.01 | 7.01 |
| Conductivity | 8.4±0.3 mS/cm | 6.65±0.03 mS/cm | 1.3 mS/cm |
| Conductivity* (pH 2) | n.d. | 12±2 mS/cm | n.d. |
| Conductivity* (pH 3) | 13.0±0.5 mS/cm | 8.5±1.0 mS/cm | 3.3±0.2 mS/cm |
| Conductivity* (pH 4) | n.d. | 8.2±0.0 mS/cm | n.d. |
| Temperature | 24±1 deg C | 27.0±0.4 deg C | 15.4 deg C |
| Redox potential | -210±10 mV | n.d. | n.d. |
| Redox potential* (pH 3) | 10±20 mV | n.d. | n.d. |
The sulfuric acid demand was higher for the sludge from Randers compared to Kolding and Haderslev (Fig 2a). For the digested sludge, a large part of the acid was consumed by the carbonate content; at Randers, the alkalinity equated to 75% of the acid demand at pH 4, 51% at pH 3 and 43% at pH 2. The conductivity increased following acidification, as expected.
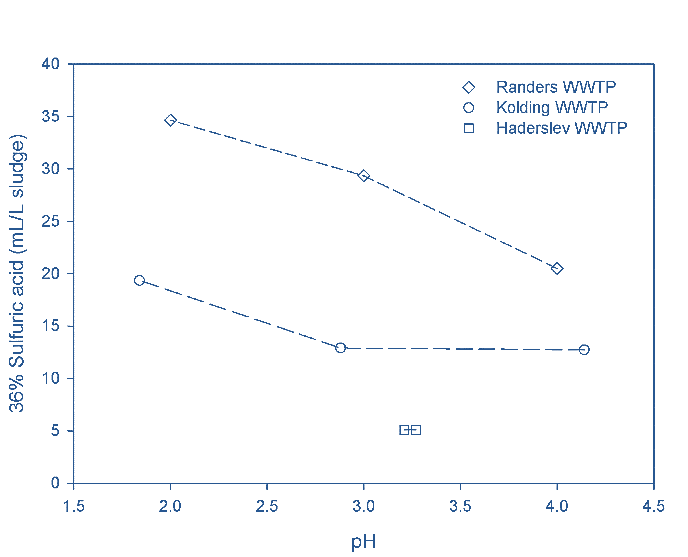
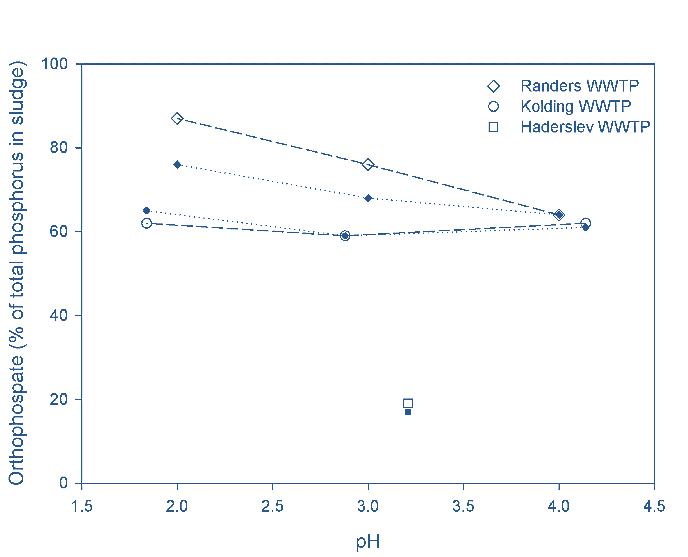
At Randers, the proportional sludge dissolved P level (as OP) increased from 8% prior to acidification to 75% at pH 3 (Fig 2b), with a similar trend at Kolding, compared to a more limited impact of acidification noted for the Haderslev sludge sample (19% released as OP at pH 3). This demonstrates the benefit of organo-P mineralisation by the anaerobic digestion process. Following dewatering, some loss of OP in the sludge cake was noted. The required acid dose was between 175 and 225 moles per kg released OP to sustain a pH of 3 for all three sites.
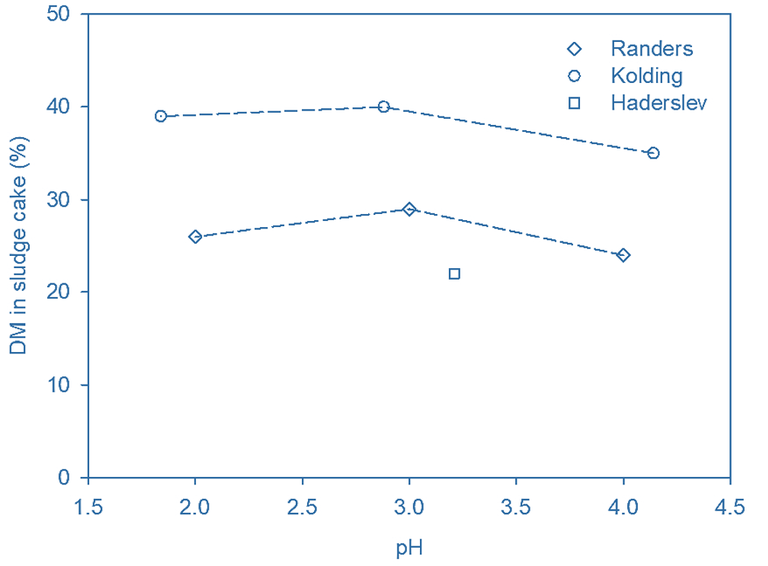
Dewatering of the acidified sludge following polymer dosing produced a cake of 20−40% DM (Fig 3a), somewhat higher than the values obtained without acidification. The DM content for untreated sludge was measured at 24−25% in both the Randers and Kolding samples, indicating an increase in sludge dewaterability resulting from acidification.
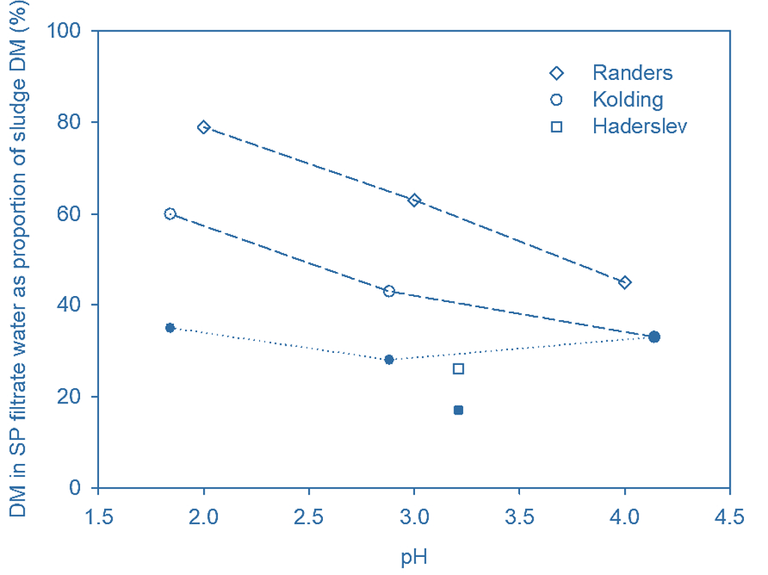
The SP filtrate DM content increased following acidification (Fig 3b), indicating a decrease in dewatering efficiency. This reflects the degradation of flocs during digestion, generating smaller organic particles and dissolved materials which are not flocculated and subsequently removed by dewatering. This then produces a turbid SP filtrate (Fig. 4); around half of the SP filtrate DM was found to be removed by introducing post-treatment rotary drum filtration (RDF), which then also removed 10–30% of the P.

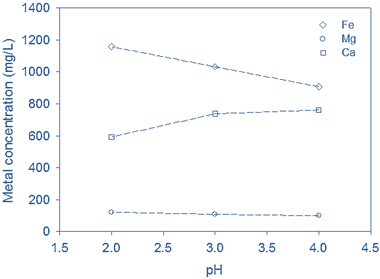
The release of iron (Fe) and magnesium (Mg) from the Randers sludge sample increased with decreasing pH, whereas the concentration of calcium declined (Fig 3c). This decrease in calcium levels may be due to the release of other anions (e.g. sulphate) at low pH which precipitate with calcium.
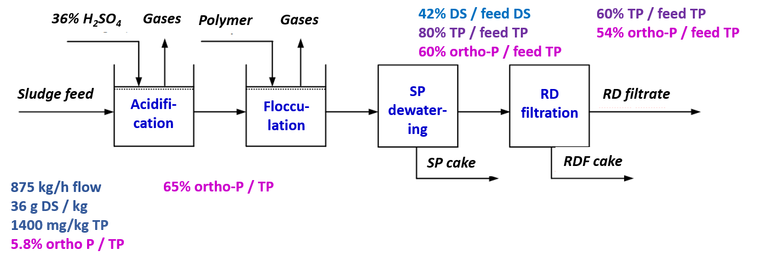
Since the largest P release was recorded for the Randers sludge (Fig. 2a), four additional pilot-scale experiments were conducted at pH 3 using this source. During acidification 36±4 kg/h of 36 % H2SO4 was added along with 140 kg/h of flocculant solution prior to SP dewatering.
| Sample site: | 1) Raw | 2) Acidified | 3) SP filtrate | 4) Cake (screw press) | 5) RDF filtrate | 6) Cake (drum filter) |
|---|---|---|---|---|---|---|
TP Total phosphorus; OP orthophosphate; TS total solids | ||||||
| Flow (kg/y) | 875±20 | 900±30 | 960±20 | 83±3 | 770±20 | 210±20 |
| DM (g/kg) | 36±2 | 47±1 | 17.6±0.7 | 264±2 | 17.2±0.5 | 23±3 |
| TP (mg/kg) | 1380±40 | 1410±80 | 1060±40 | 3200±300 | 990±30 | 1140±100 |
| OP (mg/kg) | 80±40 | 950±80 | 790±90 | n.d. | 890±60 | n.d. |
| TS (% of feed) | − | − | 42% | 55% | 33% | 12% |
| TP (% of feed) | − | − | 80% | 21% | 60% | 19% |
| OP (% of TP) | 5.8% | 68% | 60% | − | 54% | − |
Results revealed 68% of the TP to be released as OP through acidification (Table 3), some of which was lost with the filter cake following dewatering. The supplementary downstream RDF process provided an overall recovery of 54% of the inlet P content as dissolved OP in the final filtrate.
4. Discussion
Roughly 200 moles of sulfuric acid were demanded per kg OP released, for both digested and undigested sludge. Most of the phosphorus in the digested sludge samples originates from calcium and iron phosphate. Digested sludge demanded larger concentrations of sulfuric acid than undigested sludge due to the carbonate content (i.e. alkalinity), but also generated an increased release of P.
For the Randers digested sludge, six times as much sulfuric acid was required to lower the pH to 3 compared to the undigested sludge from Haderslev. However, on reducing the pH to 3, it was possible to extract 50−70% of the phosphorus from the digested sludge whereas only 20% was released from undigested sludge. Some of the released OP was then lost with the cake product stream from screw press (SP) dewatering (Fig. 5).
Large amounts of gases were released during acidification, primarily CO2 but also some H2S. This generated foam demanding dosing with a defoamer which added to the operating cost. Mechanical foam breakers or water spraying may be viable to reduce this cost.
The acidified sludge was flocculated and separated in a screw press (SP). It was possible to obtain a DM cake content up to 10% higher than that for an equivalent cake generated without acidification. The high conductivity of the acidified digested sludge did not significantly impair flocculation, though following acidification a large fraction of the DM ends up in the liquid phase, presumably due to floc disintegration.
The proportion of P lost in the SP cake can be reduced by increasing the DM cake content (and so decreasing its liquid content). A mass balance indicates 3−8% of the liquid (and thus a commensurate proportion of the OP) ends up in the sludge cake. The amount of liquid in the cake relative to the filtrate depends on the feed and sludge cake DM. The DM content of digested sludge was higher than for non-digested sludge since the sludge was concentrated prior to digestion. Thus, the highest loss of OP was observed for digested sludge.
The DM content in the SP filtrate increased on decreasing the pH from 4 to 2. This was most pronounced for the Randers sludge, where the proportion of the DM in the filtrate increased from 40% at pH 4 to 80% at pH 2. This can be attributed to floc disintegration and subsequent release of organic materials. The DM content of the SP filtrate can be reduced up to 50% by supplementary rotary drum filtration (RDF), with the organic carbon-rich cake from this step directed to the digester. P extraction combined with limited DM co-removal appears to be optimum at a pH of ~3.
5. Conclusion
Sludge acidification increased the orthophosphate concentration in the filtrate following dewatering. Based on a series of pilot trials on P recovery by acid leaching conducted on sludge samples taken from three different WwTPs in Denmark, the digested sludge provides the highest phosphorus release. This is at the expense of a relatively high acid consumption due to the high digested sludge alkalinity levels. The dry matter (DM) content of the acidified dewatered sludge was high (20−40%), the DM content in the filtrate increasing with decreasing pH. Approximately half of the filtrate DM could be removed by introducing an additional separation step. The optimal pH for phosphorus extraction was ~3, where up to 68% of the phosphorus was dissolved. Part of the released orthophosphate was lost with the filter cake leaving a recovery of up to 60% of the original total phosphorus content of the sludge in the final filtrate.






